What are aromatic aldehydes and ketones?
Aromatic aldehydes are the compounds in which –CHO group is bonded directly to an aromatic ring. Eg.

Aromatic ketones are the compounds in which carbonyl group is bonded with either both aryl group or aryl and alkyl group. Eg.

Note: The compound in which carbonyl group is not bonded directly to the benzene ring are considered as arly substituted aliphatic aldehydes. Eg.

Preparation of benzaldehyde and acetophenone
Preparation of benzaldehyde:
1. From toluene: Benzaldehyde is prepared by oxidation of toluene with cerium oxide (CeO2) in the presence of conc. H2SO4.

2. From Rosenmund’s reduction : Benzaldehyde is obtained by reducing benzoyl chloride with hydrogen in the presence of Pd catalyst deposited in BaSO4.

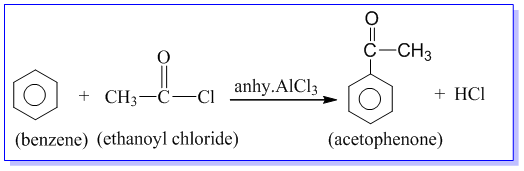
Preparation of acetophenone from benzene:
Acetophenone is prepared by the treatment of benzene with acetyl chloride in the presence of anhydrous AlCl3.
Properties of benzaldehyde
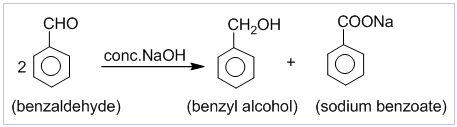
1. Cannizzaro’s reaction:
Aldehydes which do not contain α-hydrogen like HCHO, C6H5CHO,etc. undergo self oxidation and reduction on treatment with conc. alkali. In this reaction one molecule is oxidized to carboxylic acid and other molecule is reduced to alcohol. Thus, a mixture of an alcohol and a salt of carboxylic acid is formed by Cannizzaro’s reaction.
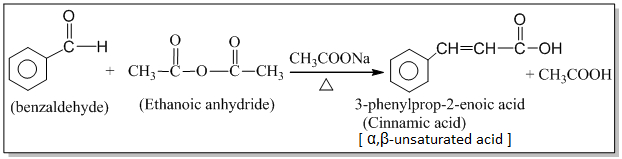
2. Perkin’s (condensation) reaction:
The condensation of an aromatic aldehyde with an acid anhydride in the presence of sodium or potassium salt of the same acid to produce α,β-unsaturated acid is known as the Perkin’s condensation.
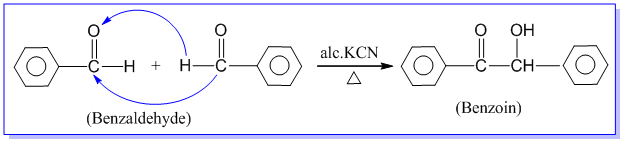
3. Benzoin condensation reaction:
Benzaldehyde when heated with alcoholic solution of potassium cyanide, undergoes self condensation between two molecules to form an α-hydroxy ketone known as benzoin. This reaction is called benzoin condensation reaction. Eg.
4. Electrophilic substitution reaction:
-CHO group is electron withdrawing group. It withdraws ∏- electrons from benzene ring, decreasing electron density of aromatic ring.
Benzaldehyde is resonance hybrid of following resonance structures:
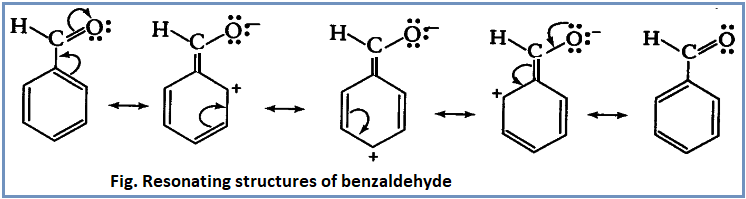
From the above resonating structures, it is clear that electron density is comparatively high at meta position. Thus incoming electrophile attacks at meta position to give meta substituted product. Thus –CHO is a meta directing group.

Similarly, acetophenone also undergoes electrophilic substitution reaction at meta position.

- Other reactions of aromatic aldehydes and ketones are similar to the reactions of aliphatic aldehydes and ketones.
- See the reactions of aliphatic aldehydes and ketones….
Post a Comment