Chemical Science Syllabus And Exam Pattern CSIR NTA NET
Council of Scientific & Industrial Research-National Eligibility Test or CSIR NET is a national level examination conducted across India to select applicants for awarding Junior Research Fellowship (JRF) and for ascertaining their eligibility for selection for lecturership (LS) in various prestigious Indian universities and colleges. Earlier the NET exam was conducted independently as UGC NET (University Grants Commission-NET) & CSIR NET. Presently, it is conducted as Joint CSIR-UGC NET exam by NTA (National Testing Agency) on behalf of the Council of Scientific & Industrial Research. CSIR NET Chemical Science exam is conducted twice in the month of June and December.
- Eligibility for CSIR NET Chemical Science Exam 2021
- Nationality – The applicant must be a citizen of India.
- Educational requirement – According to CSIR guidelines, the applicants must have at least 55% marks in BE or Integrated BS-MS or B.Pharma or B. Tech or MSc Chemistry or any other relevant science degree.
- Students who are studying M.Sc degree and have completed graduation (10+2+3) are also qualified for the CSIR NET exam.
- Maximum Age – Upper age limit for JRF is 28 years, and there is no upper age limit for LS.
- Part B: This part involves general subject-based questions from the topics provided in the CSIR NET chemical science syllabus.
- Part C: This part covers the analytical type questions based on the application of the theoretical concepts.
| General Aptitude | |
| Graphical Analysis & Data Interpretation | Pie-Chart, graph, table, graphical evaluation: Line and bar chart, mode, median, mean, and measures of dispersion. |
| Reasoning | Puzzles, Clock & calendar, series formation, Directions & distance, Ranking & arrangement, coding-decoding, etc. |
| Numerical Ability | Geometry, Time & Work, HCF & LCM average, Mensuration, Proportion & variation, Probability, Permutation & combinations, Sequence & Series, Compound interest, Simple interest, Mixture & Alligation, partnership, Trigonometry, Number & Simplification, Ratio, Time speed & distance, Profit & loss, Percentage, Quadratic equations, Logarithms, Surds & Indices, etc. |
| Overview Of CSIR NET Chemical Science Syllabus |
|
|
|
|
| CSIR NET Chemical Science Syllabus – Interdisciplinary topics |
|
|
|
|
|
CSIR NET demands in-depth knowledge of the subject besides clear concepts. For cracking the CSIR NET Chemical Science exam with excellent scores, you must definitely need to refer to good books authored by experts. Preparation is the most vital strategy to clear an exam. Adequate practice will shove your confidence to attend the examination. Books are the most reliable source for meticulous preparation. Books provide you with conceptual and application knowledge on the concerned topics. This will certainly assist you with clearing concepts, and will certainly make you a lot more knowledgeable. Likewise, you can refer to Biotecnika’s study material specially made for CSIR aspirants which covers the whole curriculum with interactive style and allows you to practice questions.
Only bonafide Indian citizens are eligible to apply for the CSIR NET exam. The exam score is accepted by universities, colleges, and research institutes in both the government and private sectors across India along with meeting the eligibility standards set by University Grants Commission.
This article is specially drafted for CSIR NET aspirants who are dreaming to wage their career in this area. This article contains a detailed note about the CSIR NET Chemical Science syllabus, and a brief note about the CSIR NET exam pattern, eligibility, important topics, and reference books.
CSIR UGC NET is conducted for subjects
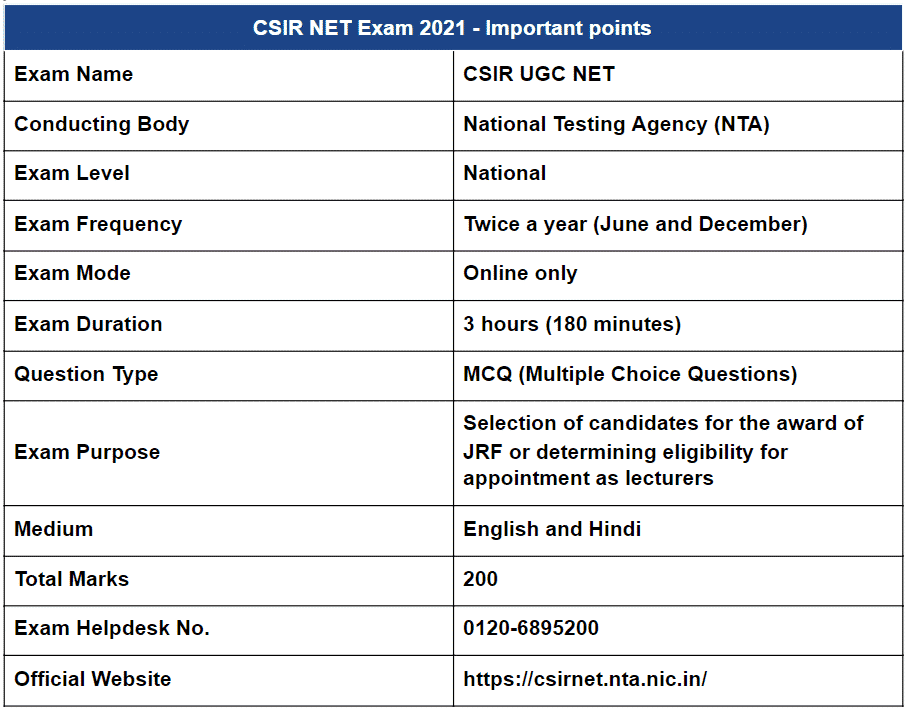
CSIR NET Exam 2021 – Important points
![]() Exam Pattern of CSIR NET Chemical science 2021
Exam Pattern of CSIR NET Chemical science 2021
Aspirants desiring to attempt the CSIR NET 2021 exam to build their career in the research or teaching profession should clearly understand the examination pattern as well as the syllabus of the CSIR NET chemical science exam. It is important to have extensive knowledge of the syllabus to get an idea of the sections asked for the exam. Exam pattern helps with easy preparation for the exam.
Part A: This part involves contains questions from general aptitude, general science skills, and quantitative reasoning & analysis, which is the same for all the subjects.
Reminder: Only the first required number of answers will be considered if the candidate answers more than the required number of questions.
Now let’s have a deeper look into the CSIR NET Chemical Science Syllabus 2021.
CSIR NET 2021 Syllabus For Chemical ScienceCSIR NET Chemical Sciences exam paper has both general aptitude and chemical science-based questions. As discussed before, a good understanding of the exam pattern and the syllabus is essential in cracking any exam.
General Aptitude (PART A)
CSIR NET exam’s Part A containing General aptitude is the same for all subjects. It mostly covers 3 areas: Graphical analysis, mathematical skills, reasoning, and data interpretation.
![]() CSIR NET Chemical Science Syllabus – 2021
CSIR NET Chemical Science Syllabus – 2021
![]() Inorganic Chemistry
Inorganic Chemistry
It is the branch of chemistry devoted to the study of the synthesis, reactions, structures, and properties of compounds of the elements. It is vital to multiple practical techniques such as energy conversion and storage, catalysis and materials, and electronics.
CSIR NET Chemical Science Syllabus – Inorganic Chemistry
i) Concepts of acids and bases, Non-aqueous solvents, and Hard-Soft acid-base concept.
ii) Organometallic compounds: bonding and structure, reactivity, and synthesis. Organometallics in homogeneous catalysis.
iii) Characterization of inorganic compounds by electron spectroscopy, microscopic techniques, Raman, IR, UV-vis, EPR, MS, NMR, Mössbauer, and NQR.
iv) Bioinorganic chemistry: porphyrins, photosystems, electron-transfer reactions, oxygen transport, metalloenzymes; metal complexes in medicine, and nitrogen fixation.
v) Nuclear chemistry: radio-analytical techniques, activation analysis, and nuclear reactions, fission, and fusion.
vi) Inner transition elements: redox chemistry, spectral and magnetic properties, and analytical applications.
vii) Chemical periodicity
viii) Cages and metal clusters
ix) Analytical chemistry – separation, electro, thermoanalytical, spectroscopic techniques.
x) Transition elements and coordination compounds: spectral and magnetic properties, bonding theories, structure, and reaction mechanisms.
xi) Structure and bonding in hetero- and homonuclear molecules, comprising shapes of molecules (VSEPR Theory).
xii) Main group elements and their compounds: industrial importance of the compounds, structure and bonding, synthesis, and allotropy.
![]() Physical Chemistry
Physical Chemistry
It is the branch of chemistry that covers the study of the behavior of matter at an atomic or molecular level. It also includes the study of the properties of substances at various ranges, from the macroscopic size which includes particles that are visible to the naked eye, to the subatomic size including very tiny subatomic particles like electrons.
CSIR NET Chemical Science Syllabus – Physical Chemistry
i) Chemical applications of group theory; point groups; symmetry elements; selection rules; character tables.
ii) Solid-state: Bragg’s law and applications; band structure of solids; and Crystal structures.
iii) Polymer chemistry: kinetics of polymerization; and Molar masses.
iv) Colloids and surfaces: isotherms and surface area; heterogeneous catalysis; and Stability and properties of colloids.
v) Atomic structure and spectroscopy; term symbols; antisymmetry principle; and many-electron systems.
vi) Data analysis: absolute and relative errors; covariance and correlation coefficient; Mean and standard deviation; and linear regression.
vii) Basic principles of quantum mechanics: Operator algebra; Postulates; tunneling; orbital and spin angular momenta; exactly solvable systems: the hydrogen atom comprising shapes of atomic orbitals; harmonic oscillator, and particle-in-a-box.
viii) Chemical kinetics: Complex reactions; determination of reaction mechanisms; steady-state approximation; homogeneous catalysis; Empirical rate laws and temperature dependence; unimolecular reactions; enzyme kinetics; collision and transition state theories of rate constants; photochemical reactions; and salt effects.
ix) Molecular spectroscopy: Basic principles of magnetic resonance; IR and Raman activities – selection rules; Electronic spectra; rotational and vibrational spectra of diatomic molecules.
x) Approximate methods of quantum mechanics: Perturbation theory up to second order in energy; Variational principle; and its applications.
xi) Elementary concepts of MO and VB theories; Huckel theory for conjugated π-electron systems; and Chemical bonding in diatomics.
xii) Electrochemistry: Ionic equilibria; DebyeHuckel theory; electrolytic conductance – Kohlrausch’s law and its applications; conductometric and potentiometric titrations; Redox systems, Nernst equation, electrochemical cells.
Organic Chemistry
It is the discipline within the subject of chemistry covering the study of structure, composition, reactions, properties, and synthesis of organic compounds that contain carbon. Organic compounds are molecules composed of carbon and hydrogen and may contain any number of other elements. Organic chemistry includes chiral synthesis, fullerene chemistry, microwave chemistry, and green chemistry.
CSIR NET Chemical Science Syllabus – Organic Chemistry
i) Common named reactions and rearrangements – applications in organic synthesis.
ii) Organic reactive intermediates: Generation, reactivity of carbocations, and stability, carbanions, nitrenes, carbenes, benzynes, and free radicals.
iii) Concepts in organic synthesis: Umpolung of reactivity, Retrosynthesis, disconnection, synthons, protecting groups, and linear and convergent synthesis.
iv) Asymmetric synthesis: Resolution – optical and kinetic. Determination of enantiomeric and diastereomeric excess; Chiral auxiliaries, methods of asymmetric induction – substrate, reagent, and catalyst controlled reactions; enantio-discrimination.
v) Synthesis and reactivity of common heterocyclic compounds containing one or two heteroatoms (O, N, S).
vi) Principles of stereochemistry: Configurational and conformational isomerism in acyclic and cyclic compounds; asymmetric induction, stereoselectivity, enantioselectivity, stereogenicity, and diastereoselectivity.
vii) Structure determination of organic compounds by 1 H & 13C NMR, IR, UV-Vis, and Mass spectroscopic methods.
viii) Chemistry of natural products: Proteins and peptides, carbohydrates, fatty acids, terpenes, steroids, alkaloids, and nucleic acids. Biogenesis of terpenoids and alkaloids.
ix) IUPAC nomenclature of organic molecules including regio- and stereoisomers.
x) Pericyclic reactions – cycloaddition, sigmatropic rearrangements, electrocyclisation, and other related concerted reactions. Organic chemistry’s principles and applications of photochemical reactions.
xi) Organic reaction mechanisms involving addition, elimination, and substitution reactions with nucleophilic, radical, or electrophilic species. Determination of reaction pathway.
xii) Organic transformations and reagents: Common catalysts and reagents (organic, inorganic, organometallic, and enzymatic); Chemo, regio, and stereoselective transformations; and Functional group interconversion including oxidations and reductions.
xiii) Aromaticity: Generation and reactions of Benzenoid and non-benzenoid compounds.
CSIR NET Chemical Science Syllabus – Interdisciplinary topics
We have listed out few books for reference for the CSIR NET Chemical Science exam.
| Reference books for CSIR NET Chemical Science |
|
|
|
|
|
|
|
|
|
|
|
|
|
![]() Important Topics to cover in priority for CSIR NET Chemical Science – Organic Chemistry
Important Topics to cover in priority for CSIR NET Chemical Science – Organic Chemistry
- Reagents
- Intermediate & Reaction Mechanism
- Pericyclic / Photochemistry
![]() Important Topics to cover in priority for CSIR NET Chemical Science from Inorganic Chemistry
Important Topics to cover in priority for CSIR NET Chemical Science from Inorganic Chemistry
- Transition Metals & Coordination Compounds.
- Main Group Elements.
- Organometallic Compounds.
![]() Important Topics to cover in priority for CSIR NET Chemical Science from Physical Chemistry
Important Topics to cover in priority for CSIR NET Chemical Science from Physical Chemistry
- Molecular Spectroscopy
- Quantum Chemistry
- Thermodynamics & Thermochemistry
- Chemical Kinetics
![]() Tips to crack the CSIR NET Chemical Science exam 2021
Tips to crack the CSIR NET Chemical Science exam 2021
- Understand the paper pattern.
- Adhere to your Timetable.
- Cover main topics at first.
- Make Short Notes.
- Work out previous year’s question papers.
- Attend Mock Tests.
- Revision is a must.
- Take regular breaks.
- Time management.
- Discover your strength and weakness.
- Use the best study materials.
- Use flow charts and diagrams while making notes, which help you while revising.
- Improve your ‘Speed and Accuracy’ by practicing questions.
- Learn topics using visual presentation and images to boost your memory power.
إرسال تعليق